About GA
Identifying GA
GA Is an Irreversible
Cause of Vision Loss1,2
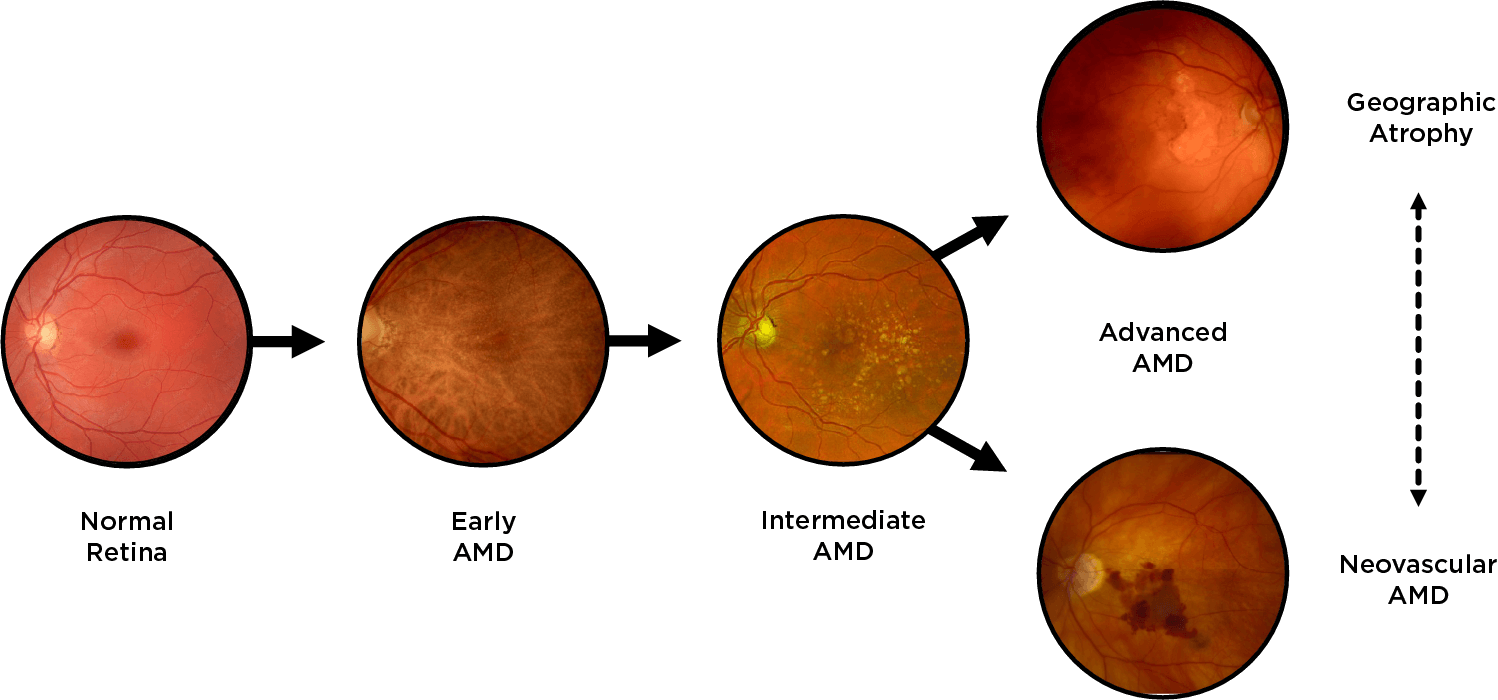
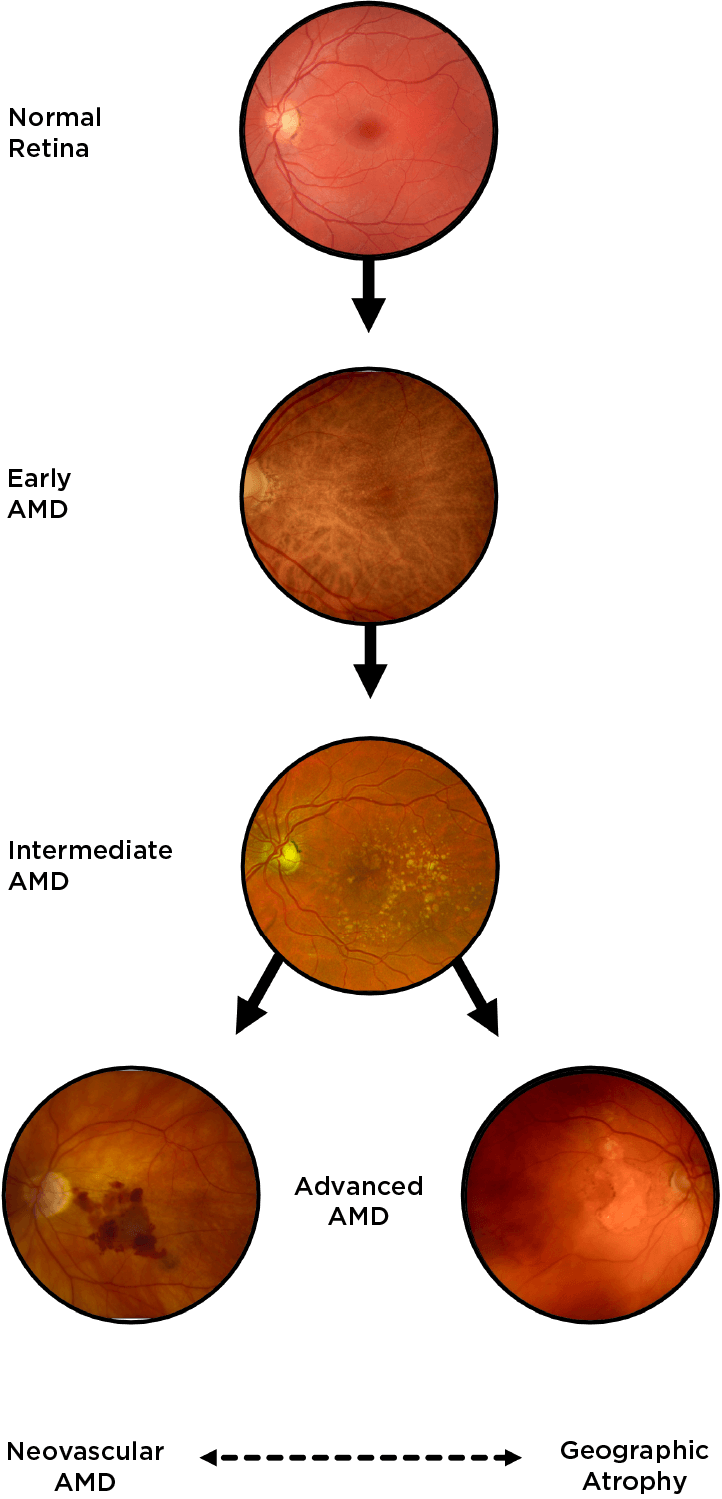
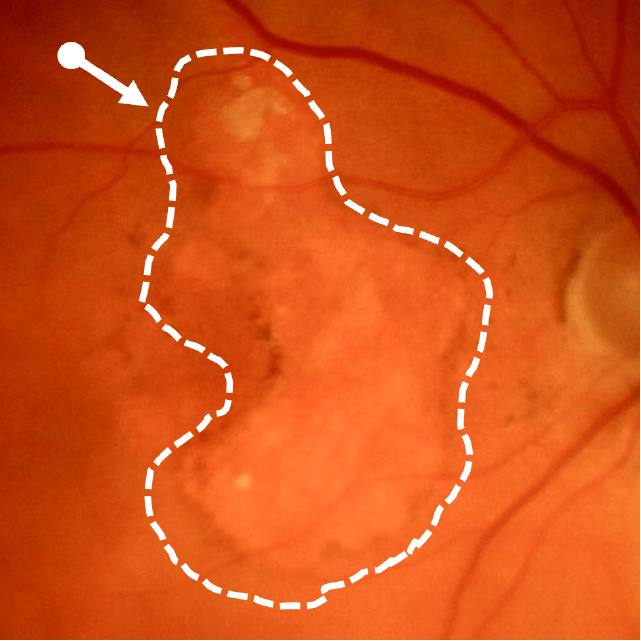
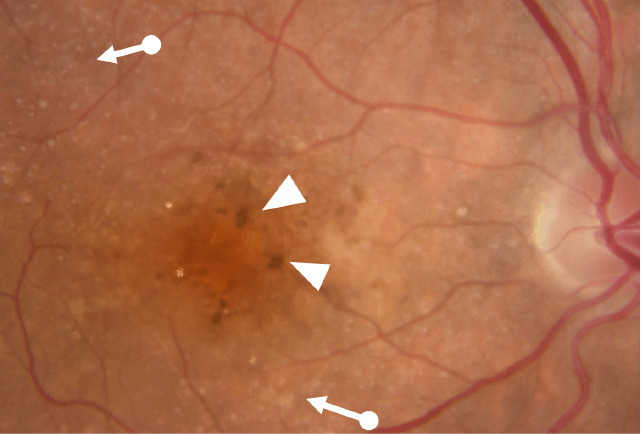
Geographic atrophy (GA), an advanced form of dry age-related macular degeneration (AMD), is defined by the presence of sharply demarcated atrophic lesions of the retinal pigment epithelium and outer retina.3,4
Drusen Are a Hallmark of Early Disease
Drusen are a hallmark of early AMD, which can be observed by direct examination on color fundus photography (CFP) or on optical coherence tomography (OCT).1,2,5,6 Drusen come in various sizes. The larger the drusen, the greater the chance of progression to an advanced form of AMD such as GA.1, 2
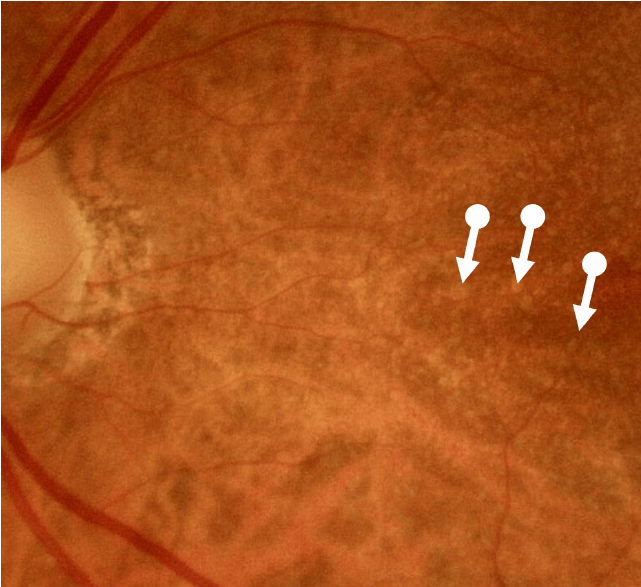
Degenerative Changes Occur in Intermediate Disease
Intermediate AMD is associated with extensive intermediate drusen (63-124 µm) or more than 1 large druse (≥125 µm).3 Pigmentary changes are also indicative of intermediate disease. Degenerative changes in the retinal layers may also be observed.3,4
Changes in visual function can occur before declines in visual acuity.7 Patients should be instructed to inform you of any sudden and/or persistent change in vision such as blurriness or distortion.8
See how disease progression impacts functional vision in the video here.
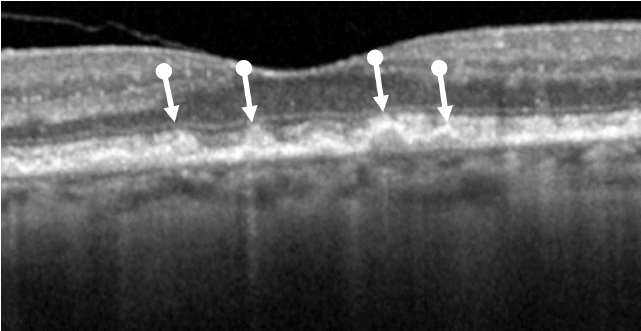
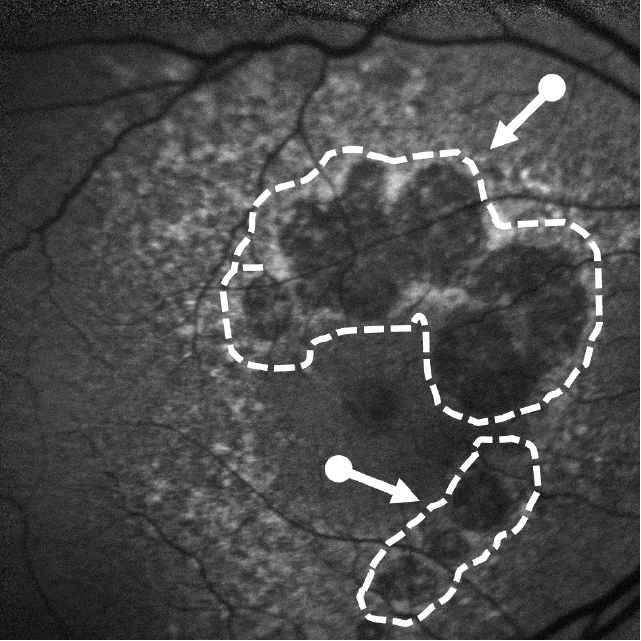
Lesion Areas Enlarge in GA
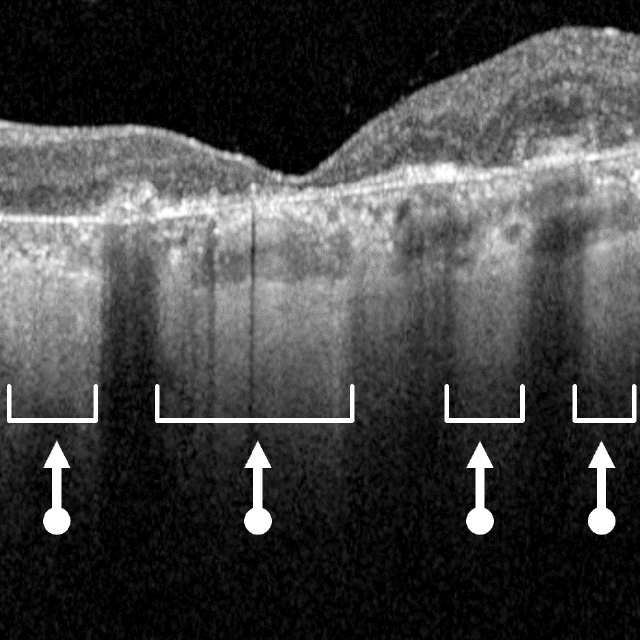
Dry AMD progression to GA is characterized by the development of new atrophic lesions, growth of individual areas, or coalescence of multiple lesions. GA can be detected using various imaging modalities, such as OCT, which are commonly available in most clinics.1-3,11
Monitoring for Progression Is Critical
Lesion patterns can be predictive of slower or faster progressing disease and provide key data to inform management strategies.2,3 Patients can present with a wide range of visual symptoms; therefore it is critical to monitor patients for disease progression.4,7
Lesion Size
Progression
Progression
Baseline Lesions
Baseline Lesions
Location
Progression
Progression
Focality
Progression
Progression
References
- Holz FG, Schmitz-Valckenberg S, Fleckenstein M. Recent developments in the treatment of age-related macular degeneration. J Clin Invest. 2014;124(4):1430-1438.
- Boyer DS, Schmidt-Erfurth U, van Lookeren Campagne M, Henry EC, Brittain C. The pathophysiology of geographic atrophy secondary to age-related macular degeneration and the complement pathway as a therapeutic target. Retina. 2017;37(5):819-835.
- Fleckenstein M, Mitchell P, Freund KB, et al. The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2018;125(3):369-390.
- Sadda SR, Chakravarthy U, Birch DG, Staurenghi G, Henry EC, Brittain C. Clinical endpoints for the study of geographic atrophy secondary to age-related macular degeneration. Retina. 2016;36(10):1806-1822.
- Mones J, Garcia M, Biarnes M, Lakkaraju A, Ferraro L. Drusen ooze: a novel hypothesis in geographic atrophy. Ophthalmol Retina. 2017;1(6):461-473.
- Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48(3):257-293.
- Sunness JS, Rubin GS, Applegate CA, et al. Visual function abnormalities and prognosis in eyes with age-related geographic atrophy of the macula and good visual acuity. Ophthalmology. 1997;104(10):1677-1691.
- Flaxel CJ, Adelman RA, Bailey ST, et al. Age-related macular degeneration preferred practice pattern®. Ophthalmology. 2020;127(1):P1-P65.
- Sadda SR, Guymer R, Holz FG, et al. Consensus definition for atrophy associated with age-related macular degeneration on OCT.Ophthalmology. 2018;125(4):537-548.
- Garrity ST, Sarraf D, Freund KB, Sadda SR. Multimodal imaging of nonneovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2018;59(4):AMD48-AMD64.
- Lindblad AS, Lloyd PC, Clemons TE, et al. Change in area of geographic atrophy in the Age-Related Eye Disease Study: AREDS report number 26. Arch Ophthalmol. 2009;127(9):1168-1174.
- Holz FG, Strauss EC, Schmitz-Valckenberg S, van Lookeren Campagne M. Geographic atrophy: clinical features and potential therapeutic approaches. Ophthalmology. 2014;121(5):1079-1091.
- Jaffe GJ, Westby K, Csaky KG, et al. Ophthalmology. 2021;128(4):576-586.
- IQVIA Medical Claims (Dx) Data Jan’20–Dec’21: 24 Months.
- Sayegh RG, Sacu S, Dunavolgyi R, et al. Geographic atrophy and foveal-sparing changes related to visual acuity in patients with dry age-related macular degeneration over time. Am J Ophthalmol. 2017;179:118-128.
- Chakravarthy U, Bailey CC, Johnston RL, et al. Characterizing disease burden and progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2018;125(6):842-849.
- Stahl A. The diagnosis and treatment of age-related macular degeneration. Dtsch Arztebl Int. 2020;117(29-30):513-520.
- Carlton J, Barnes S, Haywood A. Patient perspectives in geographic atrophy (GA): exploratory qualitative research to understand the impact of GA for patients and their families. Br Ir Orthopt J. 2019;15(1):133-141.
- Patel PJ, Ziemssen F, Ng E, et al. Burden of illness in geographic atrophy: a study of vision-related quality of life and health care resource use. Clin Ophthalmol. 2020;14:15-28.
- Singh RP, Patel SS, Nielsen JS, Schmier JK, Rajput Y. Patient-, caregiver-, and eye care professional-reported burden of geographic atrophy secondary to age-related macular degeneration. Am J Ophthalm Clin Trials. 2019;2:1-6.
- Fleckenstein M, Keenan TDL, Guymer RH, et al. Age-related macular degeneration. Nat Rev Dis Primers. 2021;7(31):1-25.
- Velilla S, Garcia-Medina JJ, Garcia-Layana A, et al. Smoking and age-related macular degeneration: review and update. J Ophthalmol. 2013;2013:895147.
- Heesterbeek TJ, Lores-Motta L, Hoyng CB, Lechanteur YTE, den Hollander AI. Risk factors for progression of age-related macular degeneration. Ophthalmic Physiol Opt. 2020;40(2):140-170.
- Curcio CA, Johnson M, Huang JD, Rudolf M. Apolipoprotein B-containing lipoproteins in retinal aging and age-related macular degeneration. J Lipid Res. 2010;51(3):451-67.
- Meleth AD, Mettu P, Agron E, et al. Changes in retinal sensitivity in geographic atrophy progression as measured by microperimetry. Invest Ophthalmol Vis Sci. 2011;52(2):1119-1126.
- Sivaprasad S, Tschosik EA, Guymer RH, et al. Living with geographic atrophy: an ethnographic study. Ophthalmol Ther. 2019;8(1):115-124.
- Xu H, Chen M. Targeting the complement system for the management of retinal inflammatory and degenerative diseases. Eur J Pharmacol . 2016;787:94-104.
- Park DH, Connor KM, Lambris JD. The challenges and promise of complement therapeutics for ocular diseases. Front Immunol. 2019;10:1007.
- Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785-797.
- Wu J, Sun X. Complement system and age-related macular degeneration: drugs and challenges. Drug Des Devel Ther. 2019;13:2413-2425.
- Coulthard LG, Woodruff TM. Is the complement activation product C3a a proinflammatory molecule? Re-evaluating the evidence and the myth. J Immunol. 2015;194(8):3542-3548.
- Kim BJ, Mastellos DC, Li Y, Dunaief JL, Lambris JD. Targeting complement components C3 and C5 for the retina: key concepts and lingering questions. Prog Retin Eye Res. 2021;83:100936.
- Xie CB, Jane-Wit D, Pober JS. Complement membrane attack complex: new roles, mechanisms of action, and therapeutic targets. Am J Pathol. 2020;190(6):1138-1150.
- Brandstetter C, Holz FG, Krohne TU. Complement component C5a primes retinal pigment epithelial cells for inflammasome activation by lipofuscin-mediated photooxidative damage. J Biol Chem. 2015;290(52):31189-31198.
- Kumar-Singh R. The role of complement membrane attack complex in dry and wet AMD - From hypothesis to clinical trials. Exp Eye Res. 2019;184:266-277.